-
 Korea.net's 24-hour YouTube channel
Korea.net's 24-hour YouTube channel- NEWS FOCUS
- ABOUT KOREA
- EVENTS
- RESOURCES
- GOVERNMENT
- ABOUT US
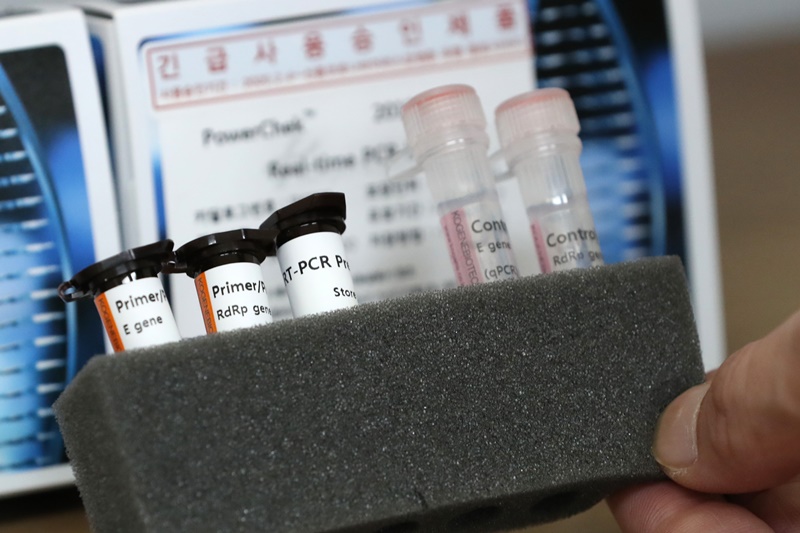
Korea is speeding up its development of diagnostic technology to swiftly respond to the novel coronavirus (COVID-19) outbreak. The photo above is a test medicine kit approved on Feb. 4 for immediate use. (Yonhap News)
By Jung Joo-ri and Lee Jihae
Korea's medical technology is playing a leading role in the government's quick response to the outbreak of the novel coronavirus disease (COVID-19), with the number of people tested reaching 105,379 on March 2.
The Korean Centers for Disease Control and Prevention (KCDC) on Jan. 31 adopted real-time RT-PCR (reverse transcription polymerase chain reaction), a testing method using DNA, at public health and environment think tanks in 18 cities and provinces. RT-PCR was adopted 18 days after the KCDC announced that it would start developing a testing method for COVID-19.
While the existing pan-coronavirus method of testing takes 24 hours and requires two stages, the RT-PCR system needs just six hours.
Since Feb. 7, the RT-PCR method has been adopted by about 50 clinics accredited by the KCDC. Private corporations in the country are also developing testing kids based on this model.
An approval system for emergency use has played a pivotal role in the government's swift response to COVID-19. Adopted in 2017 to prepare for an epidemic, the system allows the Ministry of Food and Drug Safety to temporarily approve test medicine requested by the KCDC through a fast-track procedure if a nationally certified medicine is lacking. Normally, it takes about a year for a test medicine to get approval.
The KCDC and the ministry on Feb. 4 approved the first medicines through this system.
The KCDC named test medicines from four corporations -- Seegene, Kogene Biotech, SolGent and SD Biosensor – as being used in the field as of March 4. More than 10,000 test medicines are being supplied to medical institutions conducting diagnostic tests, and another 30 medical companies await approval of their medicines.
Health authorities are focusing on swift diagnosis to find those infected with COVID-19 and reduce the sources of infection.
Central Disease Control Headquarters Deputy Director Kwon Jun-wook on March 1 told a regular briefing, "Health authorities in the U.S. also say minimization of the epidemic hinges on finding confirmed cases and cutting off the source of the virus spread."
"Quickly detecting even one confirmed case can help protect public health and reduce the potential spread of the virus even by just a little."
etoilejr@korea.kr