Humans have had wars against bacteria throughout their history. Bacteria, which is too small to be seen with the human eye, can cause various infections and even lead to death in severe cases. Researchers have developed many antibiotics to fight bacteria over the past few centuries. However, a series of superbacteria tolerant to these antibiotics has now appeared and become new threats.
Some 36,000 people pass away every year in Europe and the United States as they give in to superbacteria. The U.S. health authority has listed as many as 17 fatal superbacteria. In particular, 11,000 Americans die every year due to Methicillin-resistant Staphylococcus aureus (MRSA), a type of superbacteria.

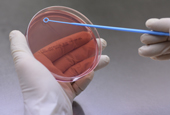
A domestic pharmaceutical company has developed a new antibacterial drug, Sivextro (tedizolid phosphate), to treat adults with skin infections caused by MRSA.
Dong-A ST, the specialty pharmaceuticals arm of Dong-A Socio Holdings, said that Sivextro was approved by the U.S. Food and Drug Administration (FDA) in June to treat adults with skin infections.
Dong-A ST spent the past 10 years developing the medicine and conducted global clinical studies on the product. Dong-A ST developed the medicine, but the U.S.-based Cubist Pharmaceuticals carried out the clinical trials. This is the second time that a drug developed by a Korean pharmaceutical company has been approved by the FDA, since Factive (gemifloxacin mesylate), an antibiotic developed by LG Life Sciences, was approved in 2003.



Sivextro is approved to treat patients with acute bacterial skin and skin structure infections (ABSSSI) caused by certain susceptible bacteria, including Staphylococcus aureus (including methicillin-resistant strains (MRSA) and methicillin-susceptible strains). Sivextro, which is available for both intravenous and oral use, cured infected patients safely within a period of time as short as six days.
In terms of safety, the chance of negative side effects on digestive organs is low. Some similar antibiotics show myelosuppressive effects, but the number of blood platelets did not go up after Sivextro was taken and it was found to be safe to use.
"Patients only need to take it once a day and the period of treatment became shorter than it used to be," said Dong-A ST President Park Chan-il. "The medicine will be available in the U.S. market beginning in late June through Cubist Pharmaceuticals. It will also be available in Europe next year when the ongoing review of the medicine by the European Medicines Agency (EMA) is completed.
By Wi Tack-whan, Limb Jae-un
Korea.net staff writers
whan23@korea.kr

Some 36,000 people pass away every year in Europe and the United States as they give in to superbacteria. The U.S. health authority has listed as many as 17 fatal superbacteria. In particular, 11,000 Americans die every year due to Methicillin-resistant Staphylococcus aureus (MRSA), a type of superbacteria.

Sivextro is an antibiotic developed in Korea to fight superbacterias.
A domestic pharmaceutical company has developed a new antibacterial drug, Sivextro (tedizolid phosphate), to treat adults with skin infections caused by MRSA.
Dong-A ST, the specialty pharmaceuticals arm of Dong-A Socio Holdings, said that Sivextro was approved by the U.S. Food and Drug Administration (FDA) in June to treat adults with skin infections.
Dong-A ST spent the past 10 years developing the medicine and conducted global clinical studies on the product. Dong-A ST developed the medicine, but the U.S.-based Cubist Pharmaceuticals carried out the clinical trials. This is the second time that a drug developed by a Korean pharmaceutical company has been approved by the FDA, since Factive (gemifloxacin mesylate), an antibiotic developed by LG Life Sciences, was approved in 2003.



Researchers at the Dong-A Socio R&D Center work on developing Sivextro, an antibiotic aimed at fighting the superbacteria MRSA.
Sivextro is approved to treat patients with acute bacterial skin and skin structure infections (ABSSSI) caused by certain susceptible bacteria, including Staphylococcus aureus (including methicillin-resistant strains (MRSA) and methicillin-susceptible strains). Sivextro, which is available for both intravenous and oral use, cured infected patients safely within a period of time as short as six days.
In terms of safety, the chance of negative side effects on digestive organs is low. Some similar antibiotics show myelosuppressive effects, but the number of blood platelets did not go up after Sivextro was taken and it was found to be safe to use.
"Patients only need to take it once a day and the period of treatment became shorter than it used to be," said Dong-A ST President Park Chan-il. "The medicine will be available in the U.S. market beginning in late June through Cubist Pharmaceuticals. It will also be available in Europe next year when the ongoing review of the medicine by the European Medicines Agency (EMA) is completed.
By Wi Tack-whan, Limb Jae-un
Korea.net staff writers
whan23@korea.kr